More news – Page 4
-
 Industry news
Industry news3D-printed device could reduce need for animal testing
A 3D-printed device is able to determine the effects of drugs on the human body and could help reduce the need for testing drugs in animals.
-
 Industry news
Industry newsNon-hormonal menopause drug approved in UK
The MHRA has authorised a non-hormonal medicine, Veoza, which targets a protein in the brain to stop vasomotor symptoms in menopause.
-
 Industry news
Industry newsEMA/HMA publish workplan for the responsible use of AI
The EMA and the HMA have published a multi-annual AI workplan 2023-2028 to facilitate the safe and effective use of artificial intelligence.
-
 Industry news
Industry newsMHRA’s IRP pathway now open for new medicines approvals
Medicines manufacturers can now submit marketing authorisation applications via the MHRA’s International Recognition Procedure.
-
 Industry news
Industry newsUS could ‘seize’ drug patents with unused Reagon-era law
The Biden administration sets new policy direction that could see the US federal government ‘seizing’ patents from publicly-funded medicines.
-
 Industry news
Industry newsFirst three countries added to WHO-Listed regulatory agencies
Singapore, the Republic of Korea and Switzerland are the first three countries to receive the status of WHO-Listed Authorities.
-
 Industry news
Industry newsProbiotic can boost immunity in children, study findings show
In a pre-clinical study, a probiotic bacteria has been found to boost immunity in newborn babies when given to the mother during pregnancy.
-
 Industry news
Industry newsMHRA eligibility checker launched for IRP framework
The MHRA has launched an online eligibility checker tool for applications submitted via its International Recognition Procedure (IRP).
-
 Video
VideoAustralian PM makes thalidomide apology on 62nd anniversary
Survivors of thalidomide receive national apology from Australian parliament − “One of the darkest chapters in Australia’s medical history”
-
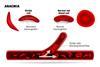 Industry news
Industry newsCRISPR tool used to treat sickle-cell and β-thalassemia
Casgevy is the first gene therapy that uses the CRISPR/Cas9 gene-editing tool to be approved by the UK MHRA.
-
 Industry news
Industry newsResearchers aim to diagnose Alzheimer’s through blood test
The Blood Biomarker Challenge will aim to determine if a blood test could be used to diagnose people in the early stages of Alzheimer’s disease.
-
 Industry news
Industry newsJ&J to submit trial application for surgical robot
Johnson & Johnson’s MedTech division is set to submit its robotic surgical system to the FDA in order to initiate clinical trials.
-
 Industry news
Industry newsCyber risk standard gets FDA backing for medical devices
The FDA has encouraged the use of a consensus standard to help device manufacturers address growing concerns over cyber security in devices.
-
 Video
VideoICMRA regulators to mark its 10th year at event in Australia
International Coalition of Medicines Regulatory Authorities set to celebrate 10 years of strategic leadership at Melbourne summit.
-
 Industry news
Industry newsWHO endorses use of R21/Matrix-M malaria vaccine in children
The World Health Organization (WHO) has recommended widespread use of the R21/Matrix-M malaria vaccine in children.
-
 Industry news
Industry newsSARS-CoV-2 can cause brain damage in dogs, says study
SARS-CoV-2 can cause changes to the brains of dogs, according to a study published in the CDC’s journal Emerging Infectious Diseases.
-
 Industry news
Industry newsPatients warned about falsified Ozempic medication
The EMA has warned patients and healthcare professionals across the EU about pre-filled injection pens falsely labelled as Ozempic.
-
 Industry news
Industry newsMHRA seeks to cut approval time by 50% for ‘lowest-risk’ RCTs
The MHRA is set to introduce a new scheme which aims reduce the time taken by the agency to approve the lowest-risk clinical trials.
-
 Industry news
Industry newsFDA to establish Digital Health Advisory Committee
The FDA has established a Digital Health Advisory Committee to help the agency explore issues related to digital health technologies (DHTs).
-
 Industry news
Industry newsSouth Africa set to investigate J&J over MDR-TB drug pricing
Johnson & Johnson is set to be investigated by South Africa’s Competition Commission for its prices charged for TB drug bedaquiline.



















