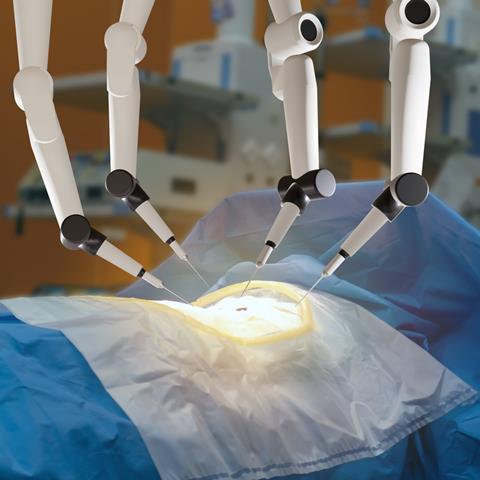
Johnson & Johnson’s (J&J) MedTech division is set to apply to the FDA for approval to begin clinical trials of its OTTAVA robotic surgical system.
Submission is planned for the second half of 2024 under the investigational device exemption. If granted, it would allow the system to be used in a clinical study to collect safety data and demonstrate its effectiveness.
The surgical robot has four flexible arms which can move in unison with the operating table. J&J MedTech said in its press release that the ‘twin motion’ of the arm and table enables surgeons to reposition patients more effectively during a procedure. The robotic arms are stored under the operating table when not in use.
“OTTAVA offers a design that incorporates into any OR and allows surgeons to do what they would like to do … focus on the patient”, said Dr Eduardo Parra-Davila, General Surgeon at the Palm Beach Digital Institute and paid consultant at J&J MedTech.
OTTAVA, if approved, would go into competition with the da Vinci Surgical System, made by Intuitive Surgical, Inc., which is the most used system worldwide with more than 90% market share for robotic surgical systems.
Further reading:
Johnson & Johnson MedTech Provides Details and Timeline for General Surgery Robot: https://www.jnj.com/johnson-johnson-medtech-provides-details-and-timeline-for-general-surgery-robot































