All Medicines and Healthcare Regulatory Agency (MHRA) articles
-
 Industry news
Industry newsMHRA eligibility checker launched for IRP framework
The MHRA has launched an online eligibility checker tool for applications submitted via its International Recognition Procedure (IRP).
-
 Industry news
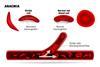
Industry newsCRISPR tool used to treat sickle-cell and β-thalassemia
Casgevy is the first gene therapy that uses the CRISPR/Cas9 gene-editing tool to be approved by the UK MHRA.
-
 Journal
JournalAccess consortium: in the current submission transmission ecosystem
The Access Consortium is a medium-sized coalition of regulatory authorities that work together to promote greater collaboration and alignment of regulatory requirements. This union began in 2007 with the involvement of Australia, Canada, Singapore, and Switzerland, and was later in 2021 bolstered by the addition of the United Kingdom’s Medicines ...
-
 Industry news
Industry newsMHRA seeks to cut approval time by 50% for ‘lowest-risk’ RCTs
The MHRA is set to introduce a new scheme which aims reduce the time taken by the agency to approve the lowest-risk clinical trials.



















