June 2025 – Contents
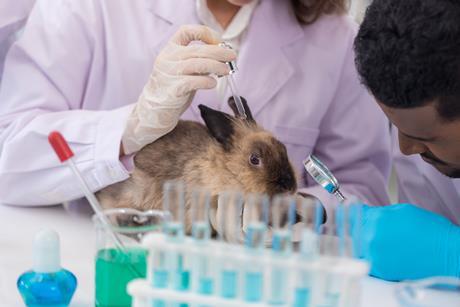
Veterinary medicines: Translating regulation into practice
Veterinary Regulation 2019/6: Impact, challenges and the future: Interview with Ivo Claassen
New European Medicines Agency fees: The effect on veterinary medicines
Promoting the use of bacteriophages in animals to reduce antimicrobial resistance
Sandbox of trust: Regulated AI for health promotion and disease prevention
Divergence on the definition of regulatory reliance in international regions: Is there room for convergence?
Medical device standards update: May 2025
- Previous
- Next
Extension to ICMRA post-approval change collaborative pilots
This industry news update looks at the collaborative pilots led by the ICMRA and other regulatory agencies.
NHS app opens access to trials for UK patients
This industry news update looks at the NHS app and the opportunities for patients to take part in clinical trials.
Updated post-market surveillance requirements take effect
This industry news update looks at the MHRA’s new PMS requirements for medical devices that took effect on 16 June 2025.
More news
MHRA provides guidance to streamline safety communication
The Medicines and Healthcare products Regulatory Agency (MHRA) has released guidance on safety communications to ensure that medicines, medical devices and other healthcare products reach ‘high standards of safety, quality and efficacy.’
Key milestone reached in African medicines regulation
A key milestone for medicines regulation in Africa has been reached with the first continental listing of five medicinal products by the African Medicines Regulatory Harmonisation (AMRH) Steering Committee.
RegRapPod − InConversation with Hugues Malonne
InConversation talks about the role of the Federal Agency for Medicines and Health Products (FAMHP) with Hugues Malonne, Chief Executive Officer at FAMHP
RegRapPod − InConversation with Ivo Claassen
InConversation talks veterinary regulation with Ivo Claassen, Head of the Veterinary Medicines Division at the EMA